Abstract
Background Pediatric immune thrombocytopenia (ITP) is an acquired autoimmune disorder which leads to a low platelet count and increased risk of bleeding. Despite published treatment guidelines, there is practice variation among clinicians and institutions due to the low grade of available evidence. The recommended management of newly diagnosed children at low risk of serious bleeding is observation; despite this, these patients are often treated with medication. These medications are only transiently effective with potential for side effects and excess lab monitoring as well as associated with increased costs and overuse of healthcare resources due to hospital admissions and encounters for medication-related side effects. Our objective is to report prospective data from a quality improvement (QI) project of the Pediatric ITP Consortium of North America (ICON) that was initiated in 2020 to improve consistency in clinical practice using national ITP guidelines.
DESIGN/METHODS Within the ICON QI subcommittee, a standardized clinical care pathway for newly diagnosed childhood ITP was developed based on the American Society of Hematology (ASH) 2019 guidelines. Sites initially completed a retrospective analysis documenting the pre-QI pathway management of children, ages 1-16 years, diagnosed with ITP from January to December 2019. After local dissemination of the clinical care pathway, clinicians at all participating sites reviewed the pathway at the time of managing newly diagnosed children and completed a short survey documenting a modified Buchanan-Adix bleeding score and management decisions. Chart review was completed six months following ITP diagnosis to collect data regarding additional treatment or hospitalization. For retrospective data, total patients include those with available data.
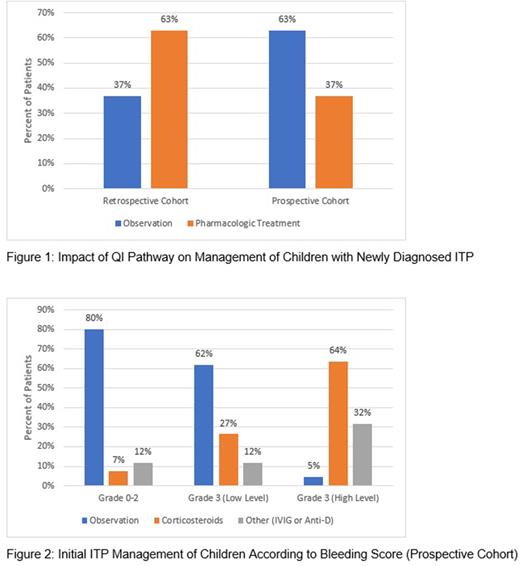
Results Retrospective data were collected from 7 centers for 205 patients managed for newly diagnosed ITP. In this pre-intervention cohort, 0.5% of patients (1/204) had a bleeding score documented at their initial visit. Most patients (63%,129/204) received initial medication treatment including intravenous immunoglobulin (54%), corticosteroids (9%), and anti-D/other (3%); 37% (75/204) were initially observed. In the 6 months after diagnosis, 11% (23/205) were admitted to the hospital for management of ITP.
The ITP QI pathway was implemented prospectively in the care of 126 patients from 7 centers between November 2020 and July 2022. Of these patients,100% (126/126) had a bleeding score documented at their initial visit [Grade 0 (2%), Grade 1 (26%), Grade 2 (27%), Grade 3 (45%), Grade 4 (0%)]. Initial treatment included close observation in the majority (63%, 79/125) and medication treatment in 37% [corticosteroids (22%, 28/125) and IVIG/anti-D (14%, 18/125)] (Figure 1). Most patients were managed per the pathway according to their bleeding score (80%, 93/117). The most common reasons that providers deviated from the pathway were parent preference (50%, 12/24) and provider concern about bleeding risk (29%, 7/24). Of those with low level grade 3 bleeding, in whom the pathway suggested close observation, 62% (21/34) were observed and 38% of patients (13/34) were treated with medication (Figure 2). For patients with high level grade 3 bleeding, in whom the pathway suggested medication treatment, all patients except one were treated with medication. Of the 66 patients with 6 month follow up data, only 2 patients (3%, 2/66) were admitted to the hospital.
DISCUSSION For children with newly diagnosed ITP and a platelet count <20 x 109/L who have no or mild bleeding, ASH guidelines suggest observation rather than treatment with corticosteroids. Implementation of an ITP clinical care pathway based on bleeding score led to an increase in the number of children observed. In contrast to the pre-intervention cohort, most patients treated with medication received corticosteroids rather than IVIG as per ASH guidelines. Despite the increase in patients managed with close observation, just 3% were admitted to the hospital within the first 6 months of diagnosis compared to 11% of the retrospective cohort. Our findings suggest that the use of a clinical care pathway based on objective assessment of bleeding symptoms increases management with close observation when indicated in the treatment of childhood ITP without a subsequent increase in hospitalization and increases adherence to national guidelines.
Disclosures
Grace:Agios Pharmaceuticals: Consultancy, Research Funding; Novartis: Research Funding; Sanofi: Consultancy; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding. Rifkin-Zenenberg:Aruvant: Honoraria; Global Blood Therapeutics: Honoraria. Lebensburger:Agios Pharmaceuticals: Consultancy; Forma Therapeutics: Consultancy; Novartis: Consultancy; BPL: Consultancy. Badawy:Vertex Pharmaceuticals Inc: Consultancy; Forma Therapeutics: Consultancy; CHIESI Farmaceutici S.p.A: Consultancy; Sanofi: Consultancy; Bristol-Myers Squibb (BMS): Consultancy; Global Blood Therapeutics: Consultancy; Pfizer Inc: Research Funding; Bluebird Bio Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal